How to Cite Fluid and Electrolyte Made It Easy
Ah, chemistry. The ephemeral fabric bringing our world together. Okay, maybe not to the majority of the population. However, nursing professionals and middle school science teachers alike must prep to understand the influence electrolytes have on this world.
So, what is important to know about electrolytes for nursing school and thereafter?
A foundational understanding of how electrolytes interact with each other and within the human body. For this resource, we will focus primarily on electrolytes. This topic often goes hand in hand with fluids (e.g. D-5W, Lactated Ringers) and acid/base balance. Maybe check back later for additional articles on these topics…or check out Picmonic for RNs or LPNs for high-quality picture mnemonics to conquer forgetting electrolyte levels, fluid types, or whatever the heck an anion gap is!
The Basics of Electrolytes
Starting off: what is an electrolyte? Electrolytes are minerals with an electric charge that can be found in your blood, urine, body tissues, other bodily fluids,1 and even outside the human body (maybe more so, but I'm not a molecular chemist, so please humor me). They serve many functions in the body:1
- Fluid and pH (acid/base) balances
- Movement of nutrients into your cells, and metabolic waste out of your cells
- Physiologic functions such as cardiac or nerve conduction
When a person eats and drinks, they consume and (hopefully) absorb electrolytes through the process of digestion.1 Once in the bloodstream and body tissues, the body regulates electrolytes' levels through physiologic activities like active transport, diffusion, and eventually excretion. Electrolytes are found inside (intracellular) and outside (extracellular) the body's cells. This fact is important to remember when thinking about specific electrolyte levels (more on this later). The most common electrolytes we will cover today are:
- Sodium (Na+)
- Calcium (Ca2+)
- Potassium (K+)
- Chloride (Cl-)
- Magnesium (Mg2+)
- Phosphate (PO43-)
In case you were wondering, the little numbers, pluses, and minuses are each electrolyte's ionic charge. For more information, check out this course from Khan Academy to buff up your knowledge coat (a little laboratory joke).
One final note: A lot of the following information was harvested fresh from the respective Picmonic Content Cards. Our ability to synthesize vast databases of information to bring you high-quality, memorable content serves as an invitation for you, Dear User, to consider diving into Picmonic for RNs or LPNs.
Holy Socratic Sodium, Batman!
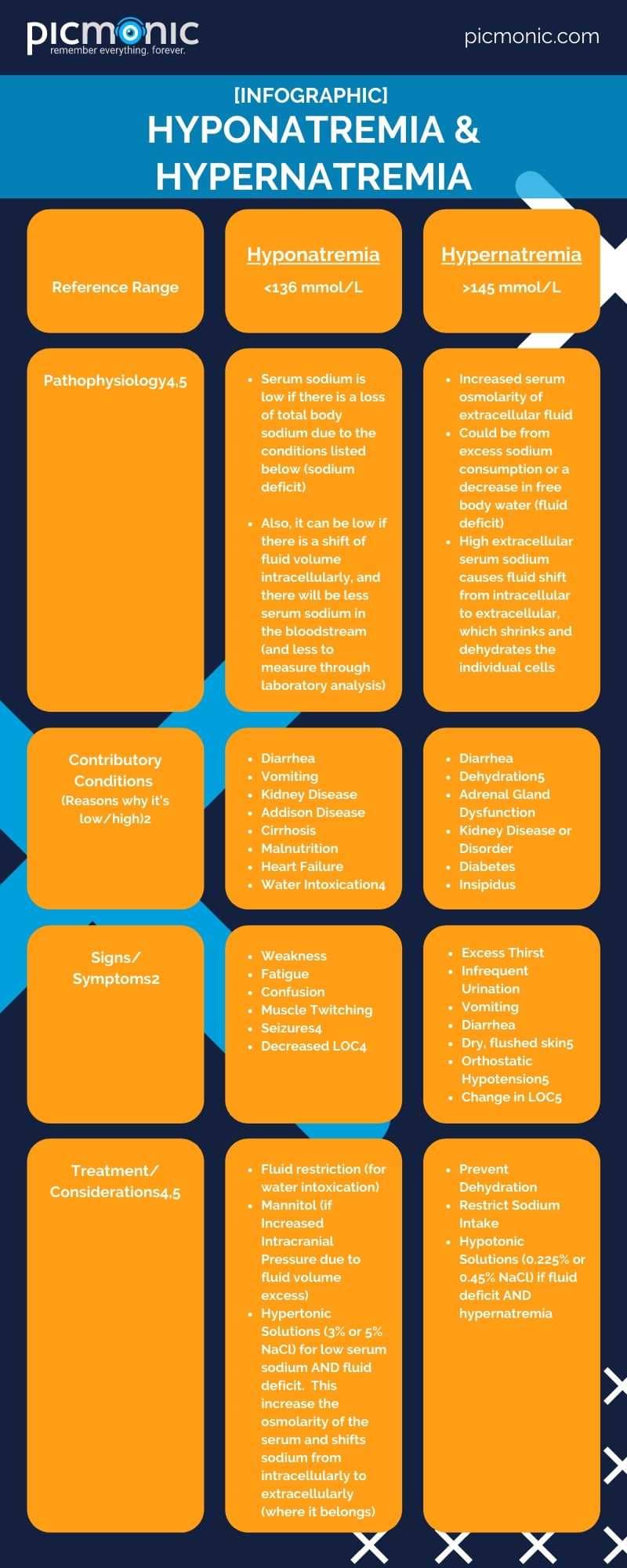
NaCL up in your salt shaker, friend! Table salt that is. For this segment, we're focusing on Na and leaving out the Cl (stay tuned). Sodium supports healthy nerve and muscle function, as well as supporting fluid balance within the body.2 Dietary intake is how humans absorb sodium (fun fact: U.S. Dietary Guidelines recommend most adults eat less than 2.3 grams of table salt per day or about 1 teaspoon), and the kidneys regulate serum (bloodstream) sodium levels under normal circumstances.2
Sodium levels can get a little wacky if there is present kidney disease, overconsumption of dietary sodium, or problems retaining fluid levels in the serum.2 These conditions can lead to low sodium (hyponatremia) or high sodium (hypernatremia) serum levels. Typically, the reference range for sodium is 136-145 mmol/L 3. Some references may cite 135 mmol/L4 as the lower reference figure. Also, serum sodium is primarily extracellular, and you'll see higher levels of sodium outside the body's cells.4
Now You See Me, Now You Calcium
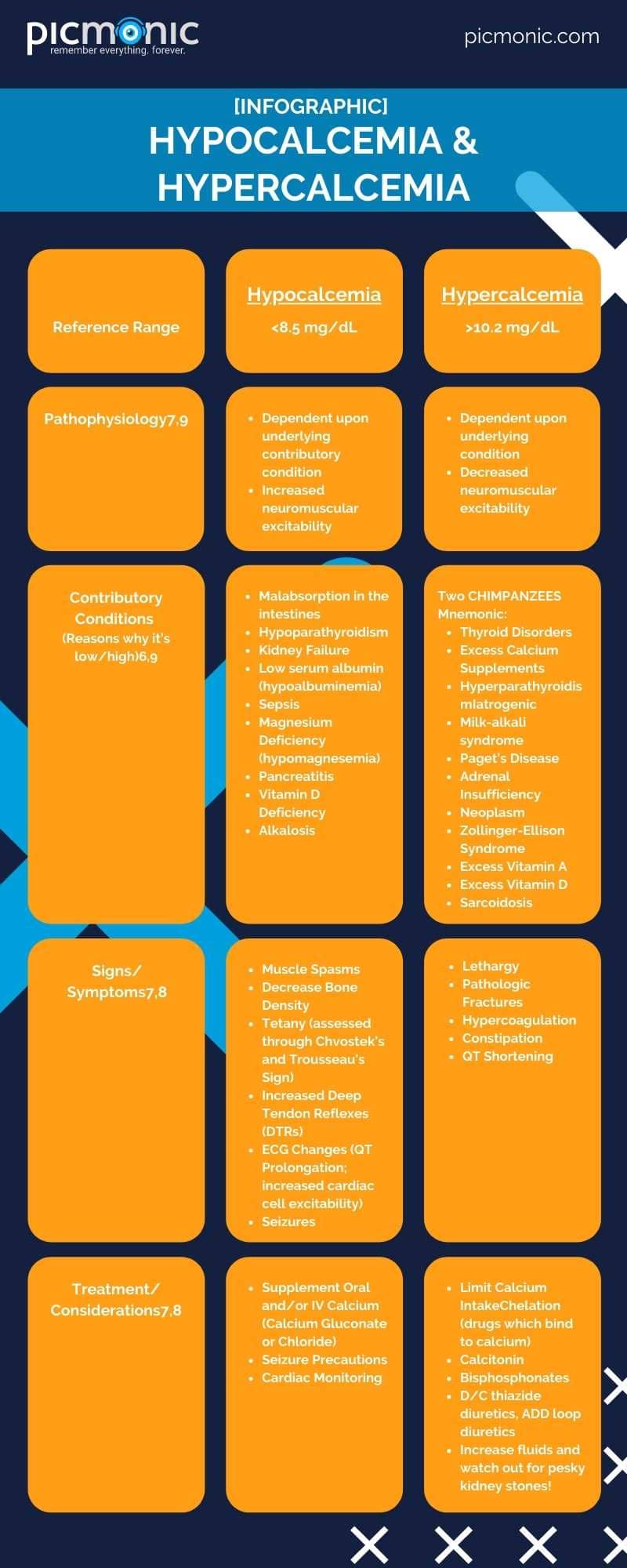
Milk does the body good! So do kale, spinach, salmon, tofu, cheese, and some fruit juices. Bones and teeth store the majority of calcium in the human body, and calcium is needed to support skeletal system development and maintenance, muscle contraction, blood vessel functions, hormone secretion, and neurologic signaling.6 Low calcium (hypocalcemia) or high calcium (hypercalcemia) levels in the serum can alter the functioning of these body systems. Serum calcium is primarily protein-bound, attaching itself for transportation around the body on albumin.6 A reference range for calcium is 8.5 to 10.2 mg/dL. Many references may present slightly different levels. A serum calcium lab measures protein-bound levels, and sometimes a provider may order an ionized calcium test, where the calcium is free or not attached to proteins.6 This measurement can help when observing changes in calcium level and diagnosis.
Potassium is BANANAS! B-A-N-A-N-A-S! (Literally)
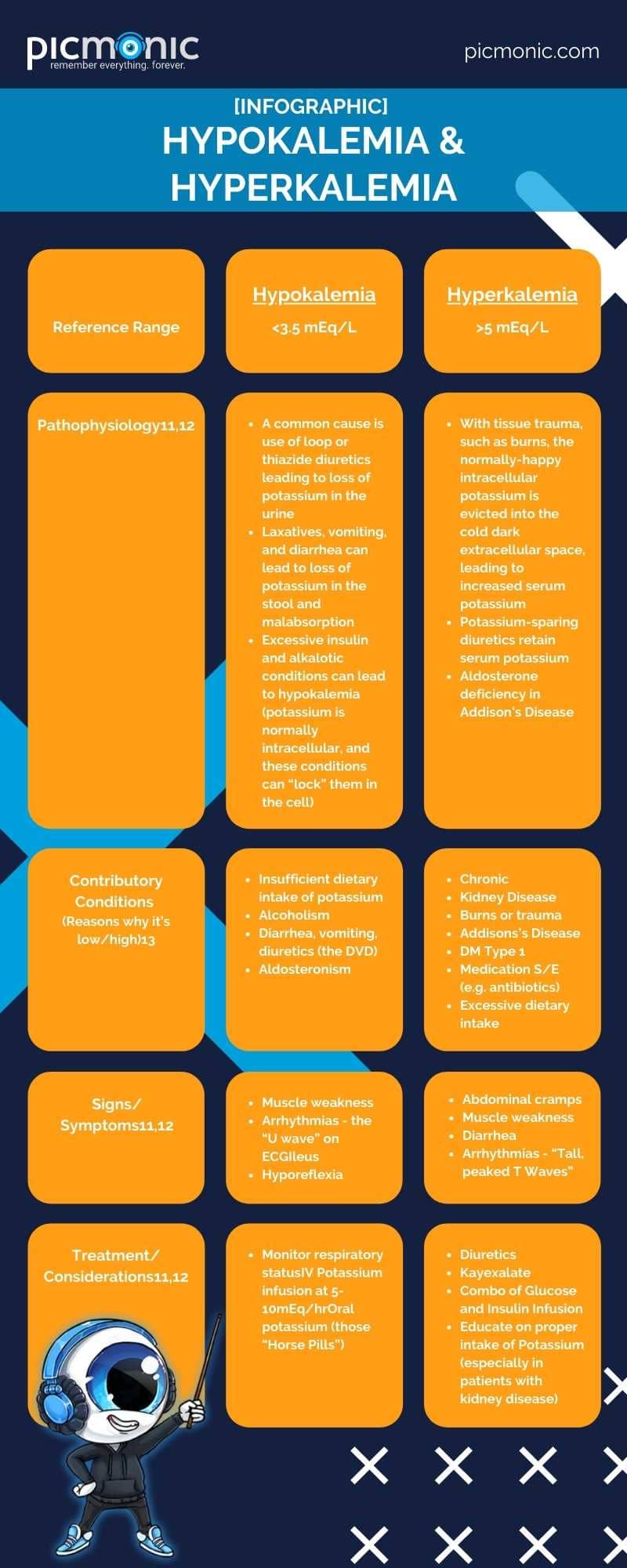
Gwen would be proud to know you are hard at work gettin' your electrolyte boogie on! The next section is all about Potassium, or K+ (the K stands for "King of the Electrolytes"). Potassium is found in spinach, bananas, collard greens, blackberries, carrots, potatoes, oranges10…the list goes on and on. As humans consume foods and drinks with potassium, their kidneys regulate potassium through the urine.10 Potassium assists with nerve, cardiac, and muscle functions and assists with bringing nutrients into the cell and escorting waste byproducts out.10 An expected range for potassium is 3.5 to 5 mEq/L11.
Intermission
This post is so long it has its own intermission! Go grab a snack, visit the porcelain throne, and let me tell you about Picmonic's new "70 Cheat Sheets for Nursing School, NCLEX and New Grads!" It's full of useful information for nursing students on Day 1 of nursing school, all the way to your 100th day as a postgraduate nurse! So, be on the lookout for this high-quality asset to your study arsenal! ….Lights flip on, then off, then on (this means returning to your seat).
Take a ChloRIDE on the Wild Side!
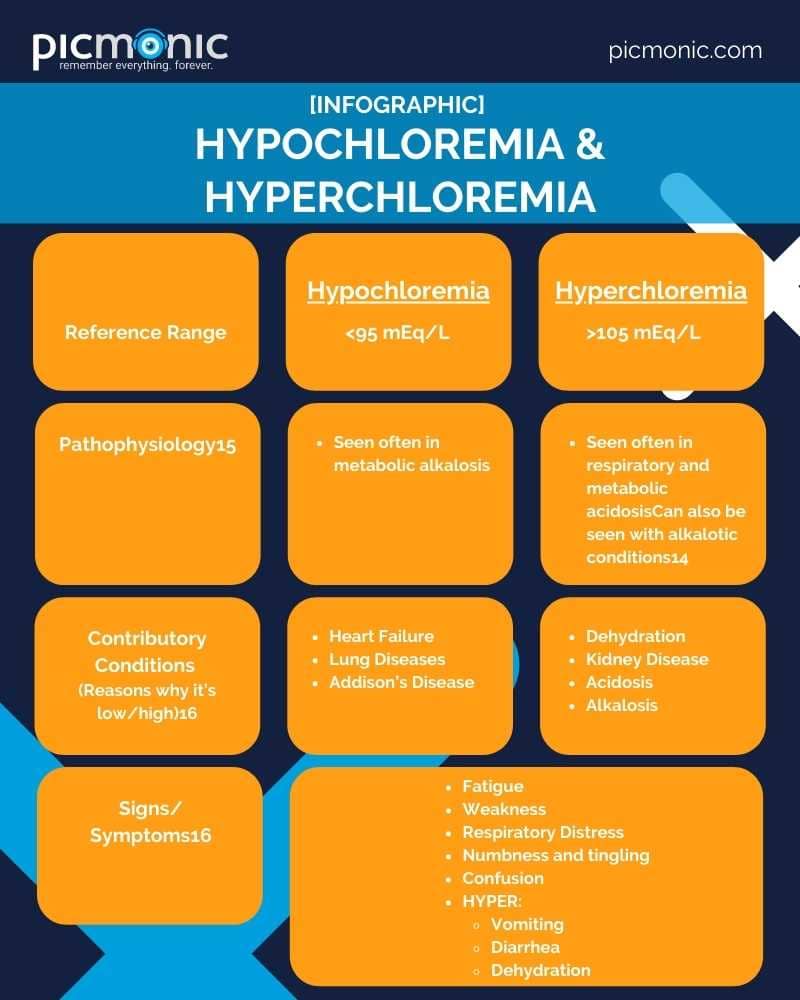
Is Chloride used in swimming pools? Close! It is one electron away! Humans consume chloride in some foods and in table salt (along with it's BFF Na+), and is needed to maintain the proper balance of bodily fluids and the composition of digestive juices.14 Also, it's important for neurons to fire, interacting with a neurotransmitter known as GABA.15The almighty kidney is what regulates the level of chloride in the serum, and a reference range to remember is 95-105 mEq/L15.
Which electrolyte has joints? MagNESium!
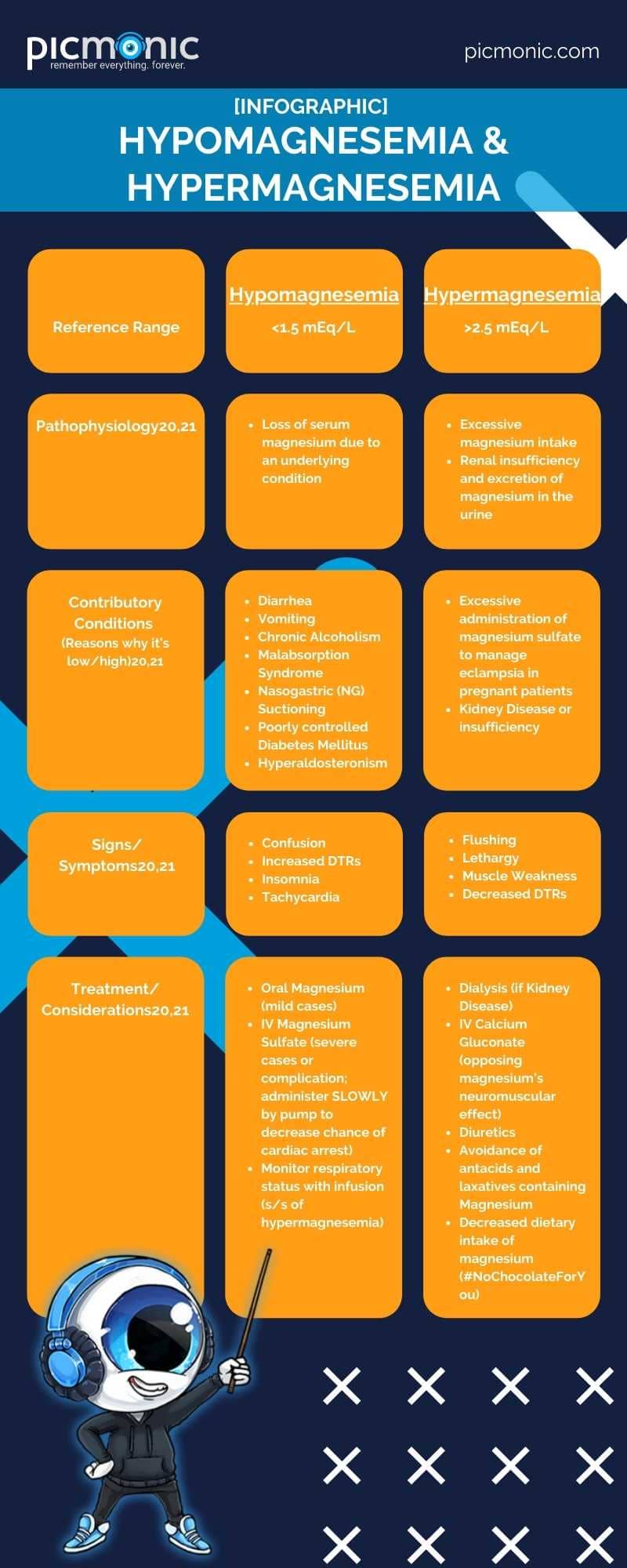
First of all, sorry for the Dad Jokes. We just can't help ourselves. Magnesium is needed for the proper functioning of your muscles, neurons and cardiac cells, and tissues.17 In fact, it's responsible for more than 300 biochemical reactions in the human body.18 It is one of the most potentially innovative electrolytes out there with ongoing research looking into its role in preventing hypertension, heart disease, and diabetes.18The reference range for magnesium is 1.5 to 2.5 mEq/L19. Magnesium is found intracellularly and as a cation with a close relationship in managing calcium and potassium balances.21 Many foods contain magnesium, including leafy, green vegetables, nuts, bananas, oranges, peanut butter, and chocolate (lots and lots of chocolate).20
Don't Hate the Phosphate!
Last dad joke…we promise. Phosphate is like a secret agent, an abundant electrolyte in your body yet secluding itself to your bones and teeth.22 Ready to strike when the moment is right. Well, ready to work with calcium to fortify your bony parts.22 Phosphate is a chemical formulation of inorganic chemicals, with a phosphorus base, and is also a key component of DNA and RNA.23 As a key component, it supports the transportation of cellular energy in ATP. The reference range for phosphate is 2.5-4.5 mg/dL.23

Closing Ceremonies
PPPPPPPHHHHHHHEEEEEWWWWWWWW!!!!
That's all folks, please enjoy this overload of information. As always, you can find much, much, much more at Picmonic for RNs and LPNs. See you on the frontlines of healthcare!
For additional reference, check out these rad infographic Resources from Picmonic!
- Hyponatremia
- Hypokalemia & Hyperkalemia
For more details, be sure to check out any of the Playlists within Picmonic for Nursing, our ready-made "Nursing Study Guides" (such as Fluid, Electrolyte and Acid-Base Imbalances), where our proven visual mnemonics will really get those concepts burned into your brain!
Download our mobile app and take Picmonic on the go!


References
- U.S. National Library of Medicine. (n.d.). Fluid and electrolyte balance. Retrieved from https://medlineplus.gov/fluidandelectrolytebalance.html
- U.S. National Library of Medicine. (n.d.). Sodium blood test. Retrieved from https://medlineplus.gov/lab-tests/sodium-blood-test/
- Le, T., Bhushan, V., Sochat, M., Schimanksy, S., Kallianos, K., Vaidyanathan, V., & Abrams, J. (2020). First aid for the USMLE step 1: 2020 (30th ed.). [Adobe Digital Editions]. Retrieved from www.mhprofessional.com.
- Picmonic. (n.d.). Hyponatremia. Retrieved from https://www.picmonic.com/learn/hyponatremia_1626
- Picmonic. (n.d.). Hypernatremia. Retrieved from https://www.picmonic.com/learn/hypernatremia_1627
- U.S. National Library of Medicine. (n.d.). Calcium. Retrieved from https://medlineplus.gov/calcium.html
- Picmonic. (n.d.). Hypocalcemia causes. Retrieved from https://www.picmonic.com/learn/hypocalcemia-causes_2507
- Picmonic. (n.d.). Hypocalcemia. Retrieved from https://www.picmonic.com/learn/hypocalcemia_1648
- Picmonic. (n.d.). Hypercalcemia. Retrieved from https://www.picmonic.com/learn/hypercalcemia_7855
- U.S. National Library of Medicine. (n.d.). Potassium . MedlinePlus. https://medlineplus.gov/potassium.html
- Picmonic. (n.d.). Hypokalemia. Retrieved from https://www.picmonic.com/learn/hypokalemia_1593
- Picmonic. (n.d.). Hyperkalemia. Retrieved from https://www.picmonic.com/learn/hyperkalemia_1594
- U.S. National Library of Medicine. (n.d.). Potassium blood test. Retrieved from https://medlineplus.gov/lab-tests/potassium-blood-test/
- U.S. National Library of Medicine. (n.d.). Chloride in diet. Retrieved from https://medlineplus.gov/ency/article/002417.htm
- Picmonic. (n.d.). Chloride (Cl-) lab value. Retrieved from https://www.picmonic.com/learn/chloride-cl-sup-sup-lab-value_1665
- U.S. National Library of Medicine. (n.d.). Chloride blood test. Retrieved from https://medlineplus.gov/lab-tests/chloride-blood-test/
- U.S. National Library of Medicine. (n.d.). Magnesium blood test. Retrieved from https://medlineplus.gov/lab-tests/magnesium-blood-test/
- U.S. National Library of Medicine. (n.d.). Magnesium in diet. Retrieved from https://medlineplus.gov/ency/article/002423.htm
- Picmonic. (n.d.). Magnesium (Mg2+) lab value. Retrieved from https://www.picmonic.com/learn/magnesium-mg-sup-2-sup-nbsp-lab-value_1666
- Picmonic. (n.d.). Hypomagnesemia. Retrieved from https://www.picmonic.com/learn/hypomagnesemia_2130
- Picmonic. (n.d.). Hypermagnesemia. Retrieved from https://www.picmonic.com/learn/hypermagnesemia_2131
- U.S. National Library of Medicine. (n.d.). Phosphate in blood. Retrieved from https://medlineplus.gov/lab-tests/phosphate-in-blood/
- Picmonic. (n.d.). Phosphate (PO43-) lab value. Retrieved from https://www.picmonic.com/learn/phosphorus-po-sub-4-sub-sup-3-sup-nbsp-lab-value_1663
I began my 14-year medical/ "Mursing" career as a Combat Medic for the Army Reserve. Shortly after completion of my BSN, I commissioned into the U.S. Army as an Army Nurse and began my tour of duty at Brooke Army Medical Center in San Antonio, TX. My adventures have brought forth a beautiful wife, three red-headed children, and two dogs (Whisky and Pancakes). We've lived in Arkansas, Texas, Kansas, Texas (yes, twice), Washington and Missouri. As of this captain's log, I'm working on completion of my BSN-DNP program at Missouri State University to become a family nurse practitioner. My prior experience and friends in medical-surgical, outpatient primary/specialized care, nursing administration, and emergency/trauma nursing (MY FAVES) motivate me to guide, mentor, teach, learn from, share bread with, play dodgeball against, and be inspired by the next generation of not only nurses and APRNs, but the entire medical and allied health community.
(Visited 8,767 times, 1 visits today)
ricardocrigoithave.blogspot.com
Source: https://www.picmonic.com/pages/electrolytes-picmonic-nursing/
